Avian Borna Virus (PDD)
Animal Genetics offers rtPCR and ELISA testing for Avian Borna Virus (ABV). ABV is believed to be the primary agent for Proventricular Dilitation Disease (PDD).
New Publication!
Description:
 Bornavirus is the causative agent of Borna disease. Borna disease virus (BDV) is a negative-stranded neurotropic RNA virus. A neurotropic virus is a virus which can infect nerve cells or which does so preferentially. A few commonly known neurotropic viruses are Rabies, Herpes virus, Poliovirus, and Japanese Encephalitis.
Bornavirus is the causative agent of Borna disease. Borna disease virus (BDV) is a negative-stranded neurotropic RNA virus. A neurotropic virus is a virus which can infect nerve cells or which does so preferentially. A few commonly known neurotropic viruses are Rabies, Herpes virus, Poliovirus, and Japanese Encephalitis.
Borna disease was first recognized in 1885 in cavalry horses in the town of Borna in Saxony, Germany. BDV, the virus that causes Borna Disease, appears to have a wide host range, including birds, horses, cattle, sheep, dogs and foxes. In 1995, the virus was isolated from cats suffering from a "staggering disease." In 2000, a Swedish researcher isolated BDV from wild birds including mallards and jackdaws. BDV has also been found in ostrich farms in Australia. It is generally accepted that BDV causes a persistent infection of the central nervous system (CNS). This infection can be expressed in varying degrees from mild behavioral changes to severe neurological disease.
Bornavirus was first described in psittaciforms in 2008 by researchers at UCSF who were studying a group of five parrots suffering from Proventricular Dilatation Disease (PDD). Using microarray technology, the group isolated a negative-stranded RNA virus from three of the five birds. This virus was determined to be a member of the Bornaviridae family. The researchers named the avian strain 'Avian Bornavirus' (ABV).
It has long been believed that PDD is caused by an enveloped neurotropic virus that in some cases causes cytokine storm resulting from lymphocyte infiltration to the proventriculus (forestomach), ventriculus (gizzard), and areas of the small intestines. As a result of the damage, birds are unable to digest their food properly. In some birds with PDD, the severely dilated thin wall of the proventriculus may rupture, resulting in movement of undigested food into the abdominal cavity causing severe infection which many times leads to rapid death.

Research strongly suggests that ABV plays a pivotal role in the onset of PDD in birds. Animal Genetics has tested over 5,000 samples from both suspect and non-suspect birds. Samples from over 500 cases of PDD diagnosed birds have been confirmed to have ABV antibodies as well as Bornavirus RNA. However, there is also a large population of birds that have tested positive for ABV but have not developed any clinical signs of PDD.
New research confirms that cloacal swabs and fecal samples are not a reliable source of ABV RNA due to the inconsistent shedding of the virus and destructive forces like bacteria, enzymes, and other contaminants found in the feces. This results in an unacceptable percentage of false negatives by PCR.
ABV has been found in samples from countries in North America, Europe, Asia, Africa and South America.
Transmission:
Bornavirus transmission is not well understood. Bornavirus is thought to be primarily transferred from one individual to another through direct or intimate contact, or by exposure to infected fecal material. Animal Genetics is currently studying vertical ABV transmission (from an infected female to an embryo in an egg) and is conducting research to determine the factors influencing embryonic infection of ABV. Additional work is being done to study ABV-specific antibody levels in the yolk of an egg laid by an infected bird.
Symptoms:
Signs suggestive of PDD include weight loss over a period of weeks to months despite a good appetite, passage of undigested food, vomiting, abdominal distention, and impaction of the crop and proventriculus. Neurological signs include intermittent shaking of the bird's head, feather plucking and mutilation, problems with balance, moaning or crying due to digestive problems, change in aggression, and seizures. Some or all of these signs may or may not be present.
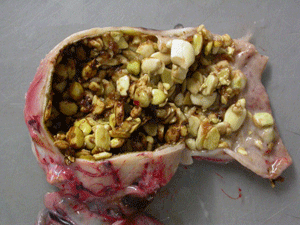
It is also important to note that in many cases birds infected with ABV may not develop any symptoms of Borna disease or PDD for years or even decades before the onset of disease. It is still unknown if a percentage of birds may never develop any symptoms of disease but may continue to function as a reservoir for ABV and allow the virus to infect other birds.
Diagnosis:
Two methods of identifying ABV infection are available at Animal Genetics: serology (using rELISA) which tests for immunological exposure to specific ABV antigens, and direct rtPCR which detects the presence of ABV-specific RNA. Our complete ABV panel includes both the rtPCR and the rELISA panel. ABV rELISA panel consists of 4 AVB-specific proteins (P40, P24, P29, and matrix). A small serum sample is required for the rELISA panel.
ABV rtPCR panel is a multiplex assay that amplifies both conserved ABV M&N segment genes as well as two internal RNA controls (housekeeping genes). Internal controls are extremely important to confirm proper RNA isolation. For routine ABV screening using rtPCR, the most reliable samples are chest or breast contour feathers or blood.
For postmortem detection of ABV RNA, samples from the brain, proventricular tissue, or crop biopsies stored in alcohol are preferred.
Animal Genetics' latest publication on ABV was submitted for publication to the Journal of Veterinarian Diagnostic Investigation. Our research paper describes current methods used by Animal Genetics for detection of ABV. This paper also demonstrates the effectiveness of testing for ABV from feathers, and compares the reliability of a feather sample with a cloacal swab and serum sample.
Sample Type:
ELISA
Two sample types are acceptable:
A) 20 microliter of blood, mixed with 250 ml of our buffer solution (0.15 M NaCl, 0.02 M Sodium EDTA pH 8.0) available from Animal Genetics Inc.
B) One drop of blood (approximately 50 microliter) on a blood card (available from Animal Genetics Inc). Air dry and seal in a plastic bag. Ensure the card is completely saturated and blood is visible on either side.
rtPCR
Cloacal swabs are not considered a reliable source for ABV RNA and should not be submitted by themselves for rtPCR.
For the rtPCR test, please submit 4-8 PLUCKED chest or breast feathers sealed in a plastic bag. Postmortem testing requires a small brain tissue sample placed in alcohol.
Limitations:
As with any genetic test, new mutations may occur in the viral genome that could affect the assay. Therefore, it may be difficult to detect all subtypes. Sample collection plays a pivotal part in the overall accuracy of the test so please use caution and follow the instructions carefully.
Publications:
Diagnosis of avian bornavirus infection in psittaciformes by serum antibody detection and reverse transcription polymerase chain reaction assay using feather calami. AH de Kloet, Kerski A, de Kloet SR. Journal of Veterinary Diagnostic Investigation. 2011 May;23(3):421-9. Source: Animal Genetics, Inc., 1336 Timberlane Road, Tallahassee, FL 32312-1766.
A Reuter, Ackermann A, Kothlow S, Rinder M, Kaspers B, Staeheli P. Avian bornaviruses escape recognition by the innate immune system. Viruses. 2010 Apr;2(4):927-38. Epub 2010 Apr 1. Source: Department of Virology, University of Freiburg, Freiburg, Germany.
Siwo R. de Kloet, Dorrestein GM. Presence of avian bornavirus RNA and anti-avian bornavirus antibodies in apparently healthy macaws. Avian Diseases. 2009 Dec;53(4):568-73.
Submit a Sample for Testing:
To submit a sample download a test submission form at Downloads
Cost per sample is $24.50-49.00. Please see our fee schedules below.